Pipeline
A robust pipeline in pursuit of transformative therapies.
We are working hard to address significant unmet need in cancer and autoimmune disease, paving the way for future treatments.
Tabelecleucel (tab-cel®)
Tab-cel® is under investigation for patients with Epstein-Barr virus-associated post-transplant lymphoproliferative disease (EBV+ PTLD) and is in earlier stage development for other EBV-associated diseases.
LEARN MORE- Indication
- Pre-clinical
- Phase 1
- Phase 2
- Phase 3
- Registration
-
-
- Tab-cel®, Atara's most clinically advanced T-cell immunotherapy in development, is currently being investigated in the Phase 3 registration-enabling ALLELE study to assess efficacy and safety for the treatment of EBV+ PTLD in SOT after failure of rituximab or rituximab and chemotherapy, and in HCT after failure of rituximab (NCT03394365, ALLELE study).
-
-
- Phase 2 multi-cohort study including EBV+ PTLD with CNS involvement, first-line treatment in PTLD, first-line treatment in PTLD with CNS involvement, EBV+ PID/AID LPD, EBV+ LMS and other potential EBV-associated diseases (NCT04554914, 205 study).
Next-generation allogeneic CAR T programs
Our focus is on applying natural T-cell biology and novel chimeric antigen receptor technologies to advance a new class of off-the-shelf CAR T-cell therapies in oncology and autoimmune disease.
ATA3219
ATA3219 is an allogeneic anti-CD19 chimeric antigen receptor (CAR) T-cell therapy, currently in clinical development, leveraging the EBV T-cell platform and features a next-generation 1XX co-stimulatory domain, memory phenotype, and unedited T-cell receptor.
LEARN MOREOncology
- Program/Indication
- Pre-clinical
- Phase 1
- Phase 2
- Phase 3
-
-
- ATA3219 is currently being investigated in a Phase 1 trial for subjects with relapsed/refractory B-cell Non-Hodgkin's Lymphoma. Initial clinical data anticipated Q1 2025 (NCT06256484).
Autoimmune
- Program/Indication
- Pre-clinical
- Phase 1
- Phase 2
- Phase 3
-
Lupus Nephritis (LN)Systemic Lupus Erythematosus (SLE) without lymphodepletion
-
-
- ATA3219 Phase 1 study initiation for subjects with lupus nephritis planned for Q4 2024. Initial clinical data expected in mid-2025 (NCT06429800).
- Initiation of ATA3219 cohort without lymphodepletion in systemic lupus erythematosus planned for Q4 2024, with initial clinical data expected in mid-2025.
ATA3431
Off-the-shelf allogeneic CD19/CD20 CAR T program progressing toward an IND submission in Q4 2025.
LEARN MORE- Program/Indication
- Pre-clinical
- Phase 1
- Phase 2
- Phase 3
-
-
- ATA3431 is an allogeneic dual CAR T candidate targeting both CD19 and CD20 antigens for B-cell malignancies.
- Dual targets reduce probability of relapse due to CD19 antigen loss, hypothesized to be a major cause of treatment resistance or disease relapse after CD19 CAR T treatment in B-cell malignancies.
- Features a novel 1XX costimulatory domain, memory phenotype, and retained, unedited T-cell receptor.
-
-
- ATA3431 is an allogeneic dual CAR T candidate targeting both CD19 and CD20 antigens for autoimmune disease.
- Features a novel 1XX costimulatory domain, memory phenotype, and retained, unedited T-cell receptor.
Additional next-generation AlloCAR T programs
We’re tackling the limitations and challenges of current CAR T therapies by developing novel next-generation allogeneic CAR T approaches.
LEARN MORE- Indication
- Pre-clinical
- Phase 1
- Phase 2
- Phase 3
-
-
- Targets: Undisclosed
- Technologies: Novel CAR T 1XX co-stimulation, memory phenotype, and retained, unedited T-cell receptor.
External Scientific Presentations
May, 2024
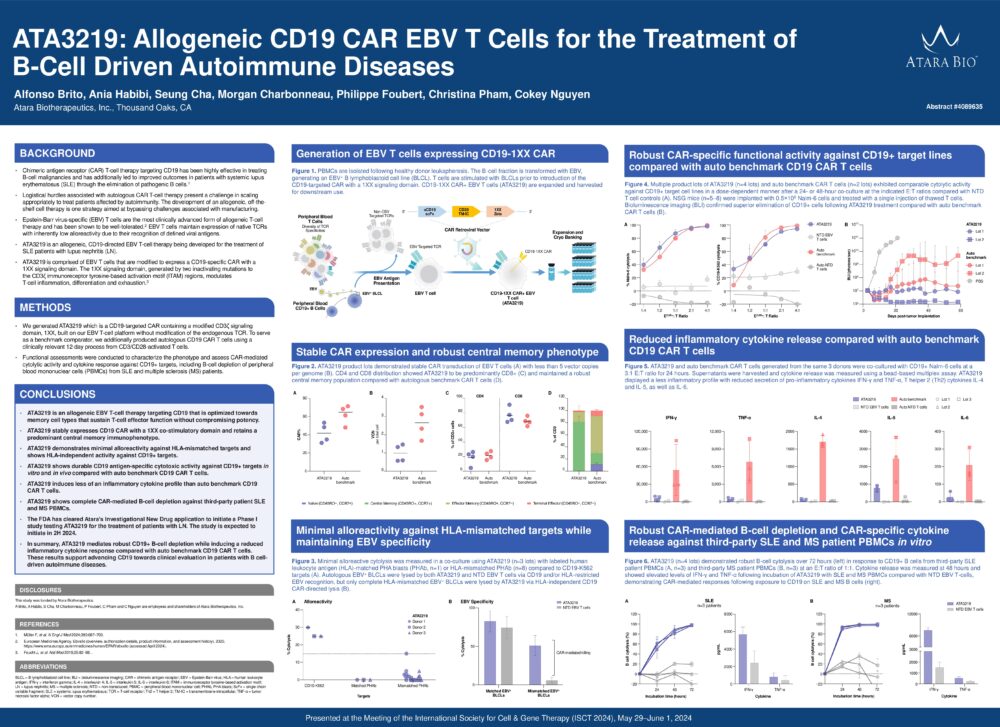
ATA3219: Allogeneic CD19 CAR EBV T Cells for the Treatment of B-Cell Driven Autoimmune Diseases
Download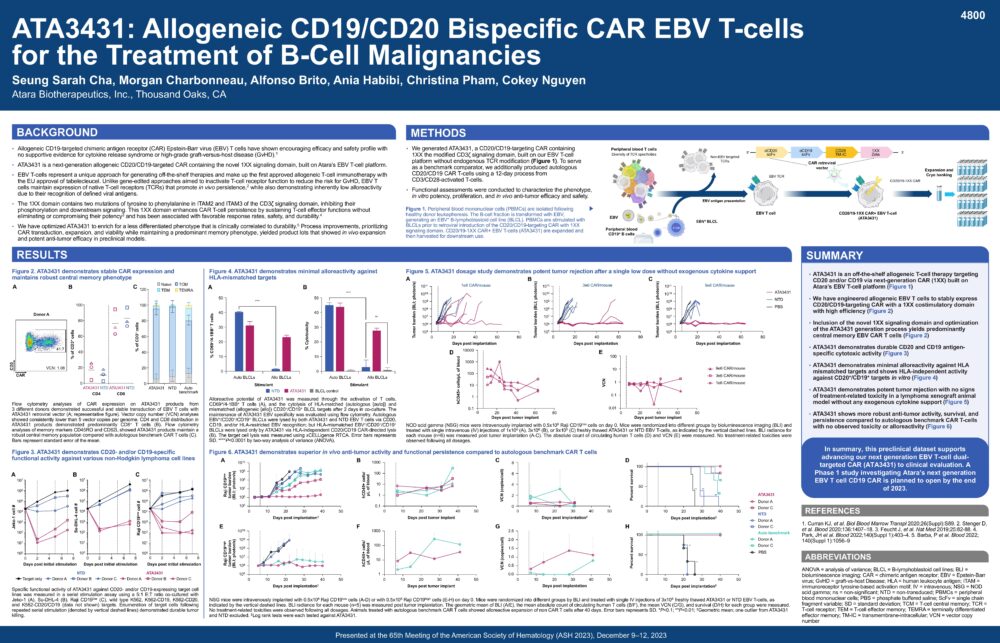
December, 2023
ATA3431: Allogeneic CD19/CD20 Bispecific CAR EBV T-cells for the Treatment of B-Cell Malignancies
Download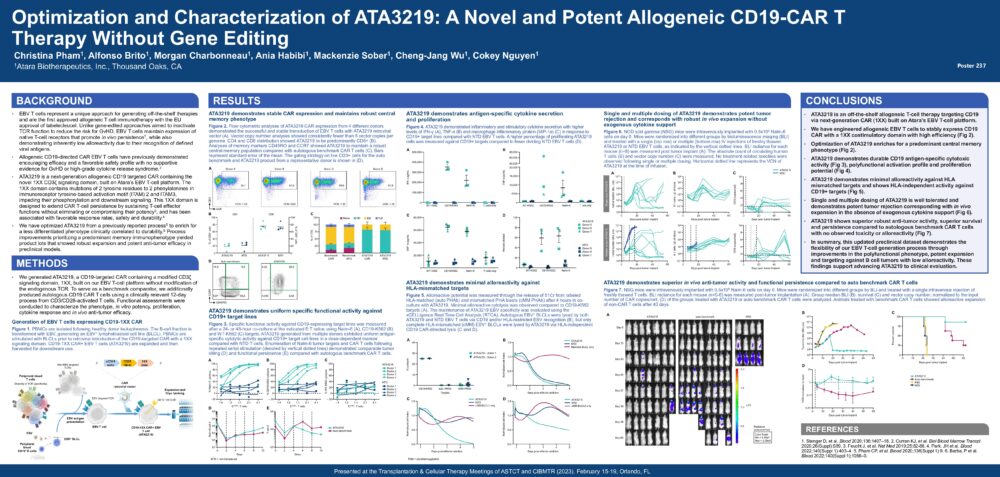
February 2023
Optimization and Characterization of ATA3219: A Novel and Potent Allogeneic CD19-CAR T Therapy Without Gene Editing
Download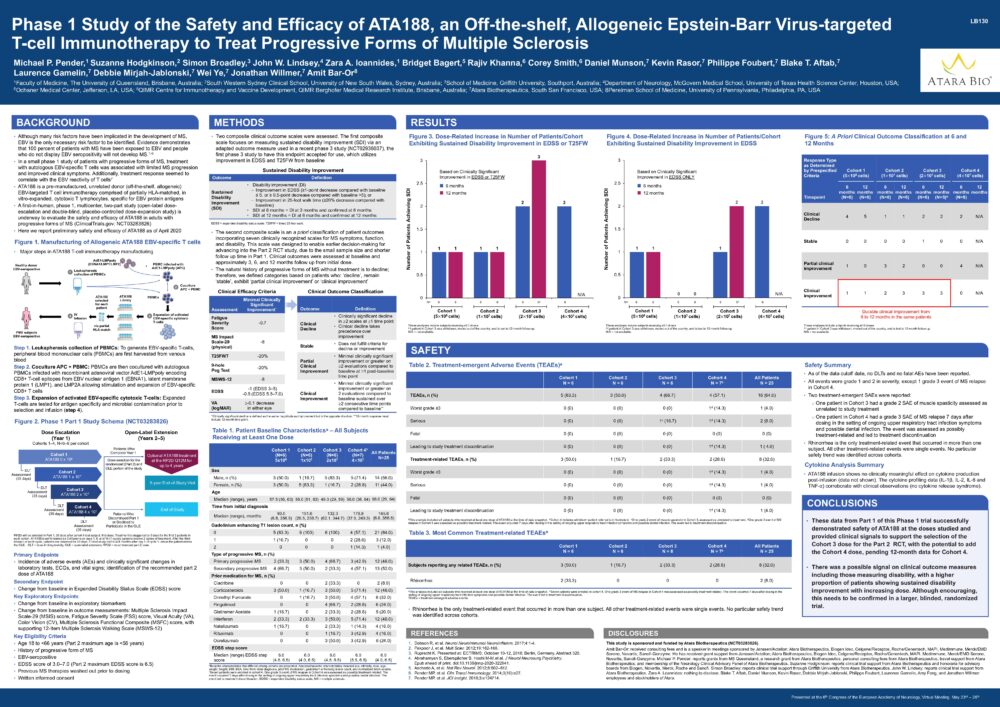
May 2020
Phase 1 Study of the Safety and Efficacy of ATA188, an Off-the-shelf, Allogeneic Epstein-Barr Virus-targeted T-cell Immunotherapy to Treat Progressive Forms of Multiple Sclerosis
Download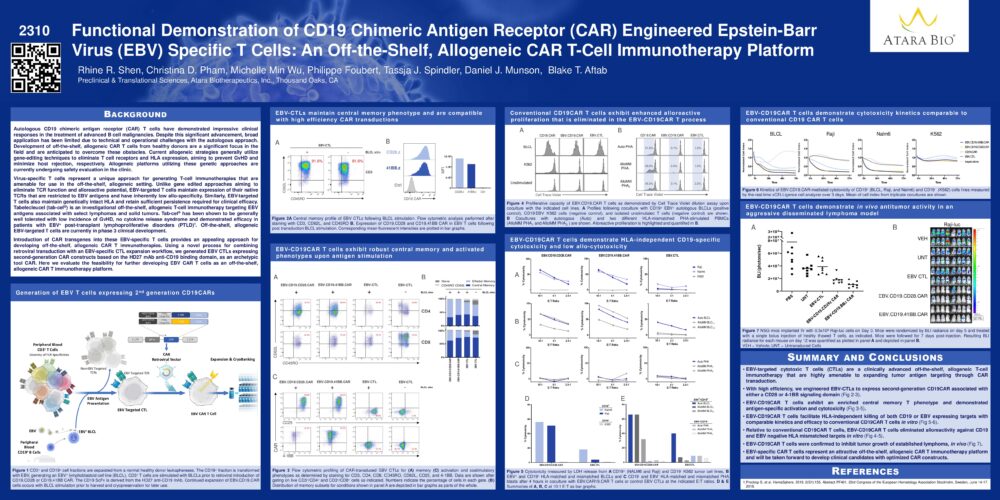
April 2019
Functional Demonstration of CD19 Chimeric Antigen Receptor (CAR) Engineered Epstein-Barr Virus (EBV) Specific T Cells: An Off-the-Shelf, Allogeneic CAR T-Cell Immunotherapy Platform
DownloadThese are investigational agents. Efficacy and safety have not been established.