Are You an Eligible Patient?
This page describes an ongoing clinical trial sponsored by Atara Biotherapeutics: A Study to Evaluate the Safety and Preliminary Efficacy of ATA3219 in Participants With Relapsed/Refractory B-Cell Non-Hodgkin Lymphoma (NHL).
You can learn more about this clinical trial, including whether you may be eligible to participate, through our clinical trial page at ClinicalTrials.gov or by reading on below.
Contact Your Doctor
Talk to your doctor about clinical trials with ATA3219 in B-cell NHL to see if you might be eligible.
Learn About B-Cell Non-Hodgkin Lymphoma
What is non-Hodgkin lymphoma?
Non-Hodgkin lymphoma (NHL) is a type of cancer that begins in your lymphatic system, which is a part of the immune system that protects the body from infection and disease. In NHL, white blood cells become cancerous and can form growths (tumors) throughout the body.
What is B-cell non-Hodgkin lymphoma?
There are many subtypes of NHL. Most subtypes begin in B cells, which normally make antibodies to help fight infections. These subtypes of NHL are known as “B-cell NHL.”
What is relapsed/refractory B-cell non-Hodkin lymphoma?
B-cell NHL is “refractory” if it does not respond to treatment. A “relapse” occurs if the B-cell NHL comes back (recurs) after it was treated. In both cases, new treatment may be needed.
References:
- National Cancer Institute. Non-Hodgkin lymphoma treatment (PDF®) – Patient version. Available from: https://www.cancer.gov/types/lymphoma/patient/adult-nhl-treatment-pdq. Accessed June 14, 2024.
- Mayo Clinic. Non-Hodgkin’s lymphoma. Available from: https://www.mayoclinic.org/diseases-conditions/non-hodgkins-lymphoma/symptoms-causes/syc-20375680. Accessed June 14, 2024.
Learn About Our Trial
What is a clinical trial?
A clinical trial is a research study that tests whether an investigational treatment is safe and effective for use in humans.
What is Atara’s ATA3219 Phase 1 trial in relapsed/refractory B-cell NHL?
This Phase 1 clinical trial is being conducted to evaluate the effects of the investigational treatment ATA3219 in participants with relapsed or refractory B-cell NHL, including B-cell NHL subtypes large B-cell lymphoma (LBCL), follicular lymphoma (FL), and mantle cell lymphoma (MCL). Participants will have disease that is relapsed or refractory to prior therapy to be eligible for inclusion. This trial is open for enrollment.
What is ATA3219?
ATA3219 is the investigational treatment being used in this Phase 1 clinical trial. This trial will test the safety and preliminary effectiveness of ATA3219 in treating B-cell NHL.
ATA3219 is an investigational chimeric antigen receptor (CAR) T cell therapy designed to treat NHL by targeting and eliminating the B cells that may be causing the disease.
Unlike some CAR T cell therapies, ATA3219 will not be made from your own T cells. Instead, ATA3219 is made from the T cells of healthy donors and stored in an inventory. Based on the molecular characteristics of the ATA3219 lots in the inventory and your immune system, a specific lot will be selected for you. This approach helps to ensure ATA3219 is available when you need it.
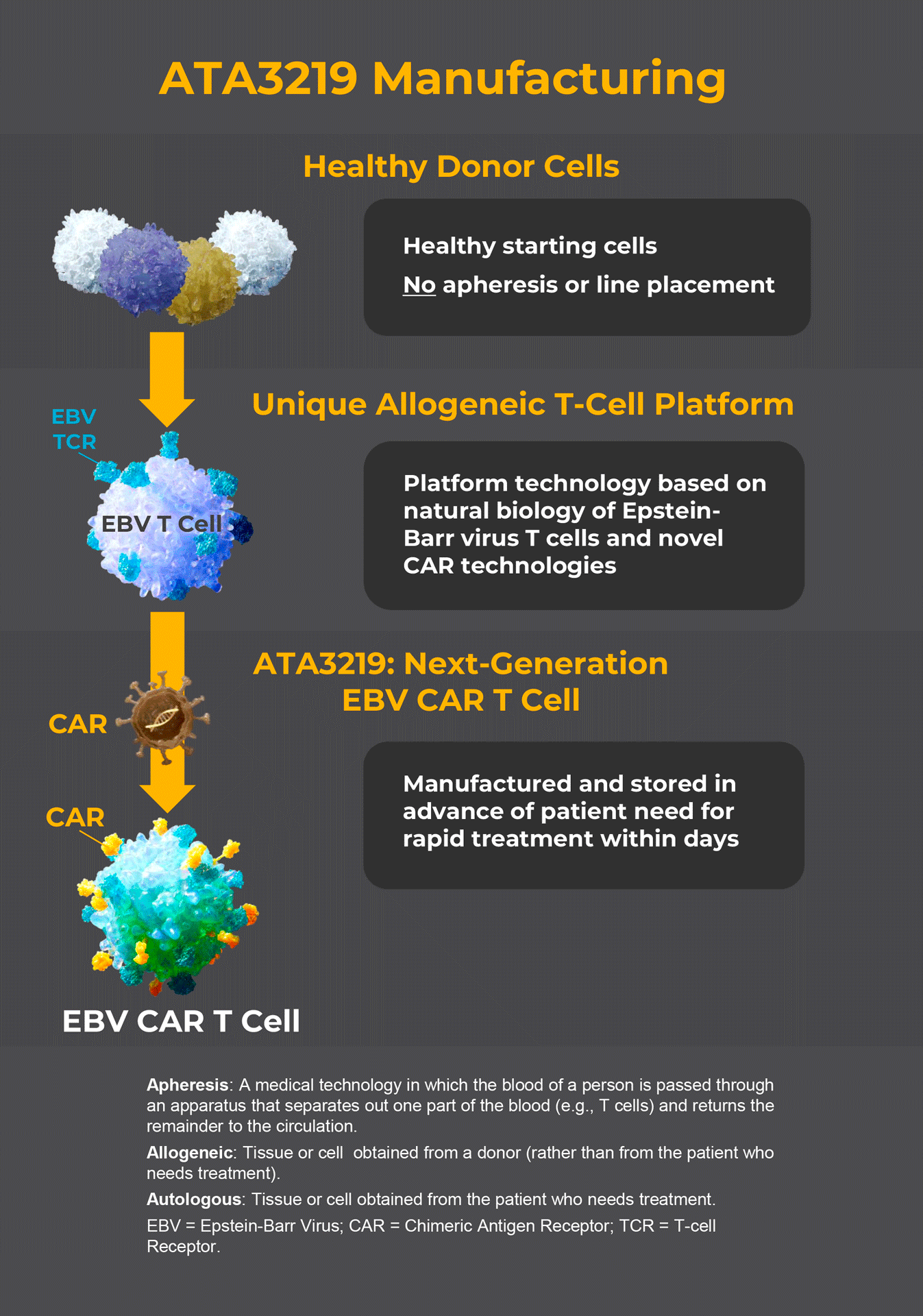
Who can participate in Atara’s Phase 1 trial of ATA3219 in B-cell NHL?
You may be eligible to participate if you:
- Are 18 years of age or older
- Have a diagnosis of B-cell NHL, including LBCL, FL (Grade 3b), or MCL
- Have measurable disease despite having tried at least two lines of therapy
- Have Eastern Cooperative Oncology Group performance status of 1 or less
- Have adequate organ function
- Are able to commit to the inpatient portion of the study, which can require therapy that helps prepare the body for infusion of the CAR T cells, frequent monitoring during the first 15 days after treatment, and remaining within 1 hour of travel time of the clinical site for 28 days after each infusion
Additional criteria will apply, and only a study doctor can determine eligibility to participate in the study. Please see ClinicalTrials.gov for more information.
Why participate in this study?
By participating in this study, you will:
- Play an important role in helping to better understand B-cell NHL
- Receive study-related care, tests, and procedures from a study doctor at no cost to you
- Receive reimbursement for reasonable travel expenses and may be compensated as determined on a site-by-site basis
What are the potential risks of participating in this trial?
Therapy with CAR T may cause serious and potentially life-threatening side effects. The potential risks of participating in this clinical trial will be explained to you before you decide to participate.
References:
- A study to evaluate the safety and preliminary efficacy of ATA3219 in participants with relapsed/refractory B-cell non-Hodgkin lymphoma. ClinicalTrials.gov identifier: NCT06256484. Updated Feb 13, 2024. Accessed June 17, 2024.
- Park JH et al. ASH 2022. Poster 163A [reference 1 in second figure option].
- Stenger D et al. Blood 2020 [reference 2 in second figure option].
- Barba P et al. ASH 2022. Poster 439 [reference 3 in second figure option].
- Pham C et al. TCT 2023. [reference 4 in second figure option].