Are You an Eligible Patient?
This page describes a clinical trial sponsored by Atara Biotherapeutics: A Study to Evaluate the Safety and Preliminary Efficacy of ATA3219, Allogeneic Anti-CD19 Chimeric Antigen Receptor T-cell (CAR T) Therapy, in Subjects with Systemic Lupus Erythematosus.
You can learn more about this clinical trial, including whether you may be eligible to participate, through our clinical trial page at ClinicalTrials.gov or by reading on below.
Contact Your Doctor
Talk to your doctor about clinical trials with ATA3219 in lupus to see if you might be eligible.
Learn About Systemic Lupus Erythematosus and Lupus Nephritis
What is systemic lupus erythematosus?
Systemic lupus erythematosus (SLE) is the most common form of lupus and is what most people mean when they refer to “lupus.” SLE is an autoimmune disease, which means that your body’s immune system attacks your own tissues and organs by mistake. This can happen when some cells in the immune system, called B cells, make proteins called autoantibodies that can attack and damage healthy tissue – including joints, skin, kidneys, blood cells, brain, heart, and lungs.
What is lupus nephritis?
Lupus nephritis (LN) is a common and serious manifestation of SLE that occurs when the kidneys are affected. Up to 60% of adults with SLE can develop kidney disease in the course of their illness. If untreated, LN can lead to kidney failure and the need for dialysis and/or a kidney transplant.
References:
- Lupus Foundation of America. National Resource Center on Lupus. Available from: https://www.lupus.org/resources/what-is-systemic-lupus-erythematosus-sle . Accessed June 17, 2024.
- Mayo Clinic. Lupus. Available from: https://www.mayoclinic.org/diseases-conditions/lupus/symptoms-causes/syc-20365789. Accessed June 17, 2024.
- Yo JH et al. Open Access Rheumatol. 2019;11:179–88.
- Neves A et al. Res Rep Urol. 2023;15:333–53.
- Yap DY et al. Nephrol Dial Transplant. 2012;27:3248–54.
Learn About Our Trial
What is a clinical trial?
A clinical trial is a research study that tests whether an investigational treatment is safe and effective for use in humans.
What is Atara’s ATA3219 Phase 1 trial in Systemic Lupus Erythematosus?
This Phase 1 clinical trial is being conducted to evaluate the effects of the investigational treatment ATA3219 in participants with lupus nephritis (LN) following lymphodepletion (LD) and in participants with extrarenal systemic lupus erythematosus (SLE) without LD. The trial is expected to initiate and start enrolling participants in late 2024.
What is ATA3219?
ATA3219 is the investigational treatment being used in this Phase 1 clinical trial. This trial will test the safety and preliminary effectiveness of ATA3219 in treating SLE.
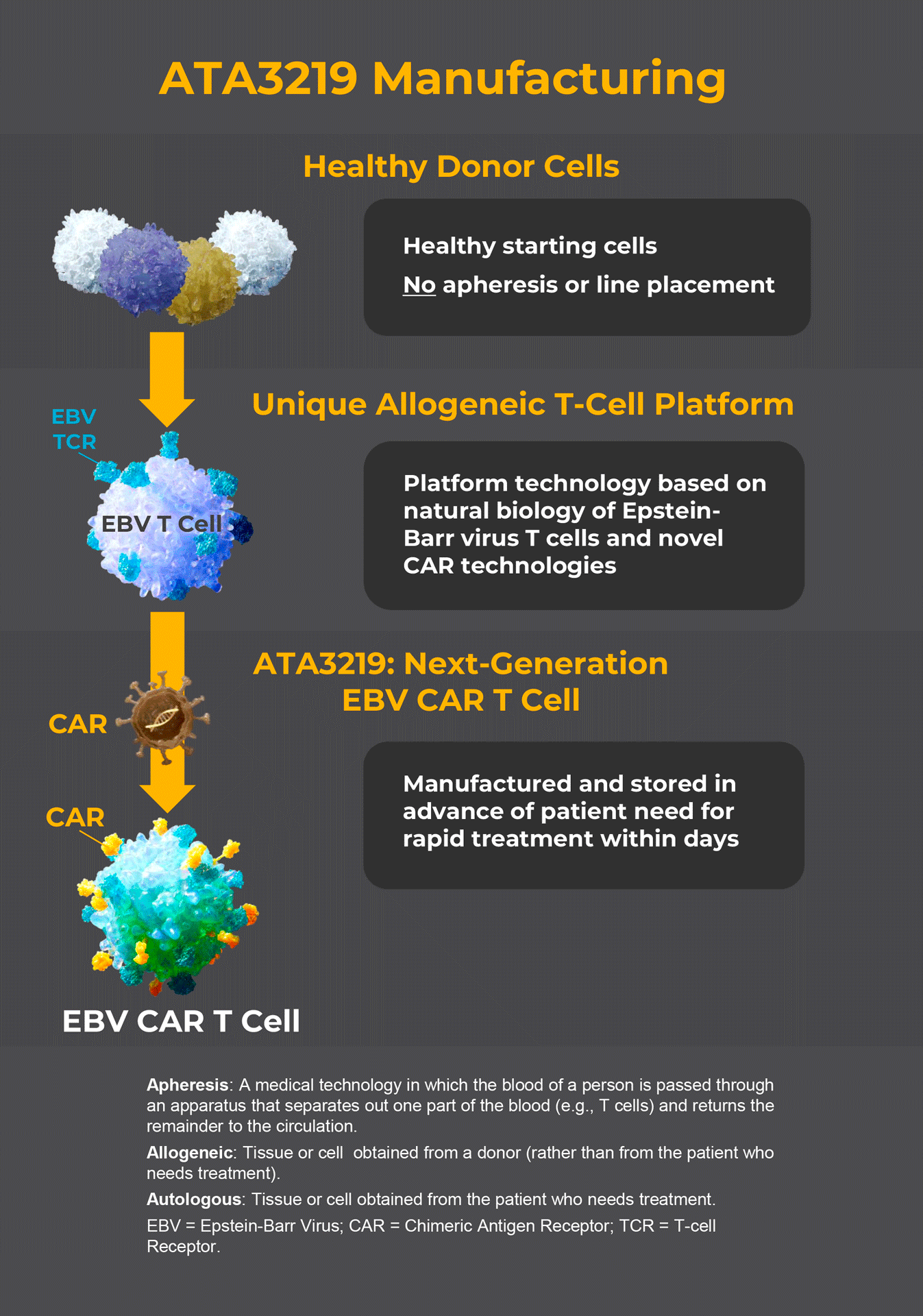
ATA3219 is an investigational chimeric antigen receptor (CAR) T cell therapy designed to treat LN by targeting and eliminating the B cells that may be causing the disease.
Unlike some CAR T cell therapies, ATA3219 will not be made from your own T cells. Instead, ATA3219 is made from the T cells of healthy donors and stored in an inventory. Based on the molecular characteristics of the ATA3219 lots in the inventory and your immune system, a specific lot will be selected for you. This approach ensures ATA3219 is available before you need it, and it also minimizes the need for inconvenient procedures that are required when CAR T treatments are generated from patient T cells.
BLCL = B-lymphoblastic cell line; CTL = cytotoxic T cell; EBV = Epstein–Barr virus; HLA = human leukocyte antigen; scFv = single-chain variable fragment; TCR = T-cell receptor; TM-IC = transmembrane – intracellular.
Who can participate in Atara’s Phase 1 trial of ATA3219 in SLE?
You may be eligible to participate if you:
- Are a male or female between 18 and 55 years of age
- Have a diagnosis of SLE
- Meet predetermined immunologic criteria at screening
- Adequate lung, liver, kidney, and cardiac function
For the Lupus Nephritis Cohort:
- Patients with SLE, class III-IV +/- class V LN
- Refractory LN in patients having received one or more lines of therapy for LN
For the Extrarenal Systemic Lupus Erythematosus:
- Patients with SLE without severe kidney disease
- Meet BILAG-2004 criteria and a qualifying PGA score
- Hybrid SELENA-SLEDAI score of ≥ 8 points at screening
Additional criteria will apply, and only a study doctor can determine eligibility to participate in the study. Please see ClinicalTrials.gov for more information.
Why participate in this study?
By participating in this study, you will:
- Play an important role in helping to better understand lupus
- Receive study-related care, tests, and procedures from a study doctor at no cost to you
- Receive reimbursement for reasonable travel expenses and may be compensated as determined on a site-by-site basis
What are the potential risks of participating in this trial?
Therapy with CAR T may cause serious and potentially life-threatening side effects. The potential risks of participating in this clinical trial will be explained to you before you decide to participate.
References:
- A study to evaluate the safety and preliminary efficacy of ATA3219 in participants with lupus nephritis. ClinicalTrials.gov identifier: NCT06429800. Updated May 28, 2024. Accessed June 17, 2024.
- Park JH et al. ASH 2022. Poster 163A [reference 1 in second figure option].
- Stenger D et al. Blood 2020 [reference 2 in second figure option].
- Barba P et al. ASH 2022. Poster 439 [reference 3 in second figure option].
- Pham C et al. TCT 2023. [reference 4 in second figure option].